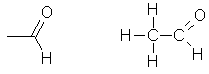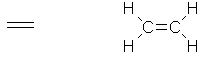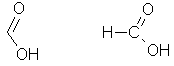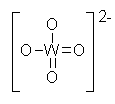|
Still not found it? Try… |
|
Click on an orange label to see a hazcard Click on certain words in bold to see another dictionary entry |
|||
|
Another way
of saying ethanal. |
|
|
|
|
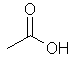
The
old-fashioned name for ethanoic acid, which is found in vinegar. |
|
CH3COOH Also: C2H4O2 |
|
|
Another
way of saying ethyne. |
|
|
|
|
Another
way of saying acyl
chloride. |
|
|
|
|
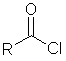
A compound
containing the group RCOCl. The acyl chloride group is similar to the
carboxylic acid group, except that the hydroxyl group has been replaced with
a chloride group. Acyl chlorides are
highly reactive. |
|
RCOCl |
|
|
alkanes (homologous series) |
Hydrocarbons
containing only carbon-carbon single bonds and carbon-hydrogen bonds |
|
CnH2n+2 |
|
(homologous series) |
Hydrocarbons
containing one or more carbon-carbon double bonds |
|
CnH2n |
|
alkynes (homologous series) |
Hydrocarbons
containing one or more carbon-carbon triple bonds |
|
CnH2n-2 |
|
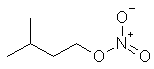
A
colourless liquid added to diesel to improve ignition. |
|
C5H11ONO2 |
|
|
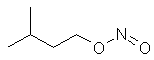
A yellowish
volatile synthetic liquid used medicinally as a vasodilator (a drug that
makes blood vessels dilate) and inhaled as a recreational drug. Sometimes
it is called amyl nitrate, but this misleading. Amyl nitrate is used in diesel. |
|
C5H11ONO |
|
|
A volatile
liquid hydrocarbon present in coal tar and petroleum, having a hexagonal
ring-shaped molecule which is the basis of most aromatic organic compounds. The
simplest aromatic compound, a member of the arene homologous series, a known
carcinogen (cancer causing chemical) and a constituent of petrol in the Origin of name: benzoic acid
+ ene
(denoting double bonds). Benzoic acid
is a white crystalline compound present in the plant resin benzoin. Benzoin is a fragrant gum resin obtained from certain East Asian storax trees. A
crystalline aromatic ketone present in this resin
is also called benzoin, from which benzoic acid
takes its name. Benzoin the resin is named from the French benjoin from the Arabic lubānjāwī
meaning incense of Java. |
|
C6H6 |
|
|
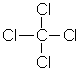
AKA
tetrachloromethane or carbon tetrachloride, this anaesthetic liquid can be
fatal in overdose. |
|
CCl4 |
|
|
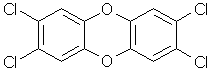
Carcinogenic
polluting compound, full name 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). |
|
C12H4Cl4O2 |
|
|
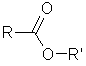
A molecule
made from an alcohol and a carboxylic acid.
The link between the alkyl groups of the two constituent molecules is
called an ester link. |
|
RCOOR |
|
|
The
aldehyde that is based on ethane. It has
two carbon atoms, one with three hydrogen atoms attached, and the other
doubly bonded to an oxygen atom and singly bonded to a hydrogen atom. Also
called acetaldehyde. |
|
CH3CHO |
|
|
ethanoic acid |
A
carboxylic acid formed by the oxidation of ethanol. |
|
CH3COOH Also: C2H4O2 |
|
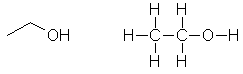
The
alcohol based on ethane. Click here for loads more detail. |
|
CH3CH2OH Also: C2H5OH or C2H6O |
|
|
A
colourless, highly flammable gas. |
|
C2H4 |
|
|
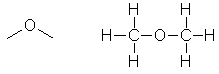
Ethoxyethane,
used as an anaesthetic. |
|
CH3OCH3 |
|
|
(homologous series) |
Organic chemicals
whose molecules consist of two alkyl groups bonded to either side of an
oxygen atom. |
|
ROR’ |
|
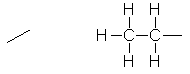
An alkyl
group consisting of –CH2-CH3 (–C2H5). It’s basically just ethane with one
hydrogen atom removed. |
|
C2H5- |
|
|
An
old-fashioned name for ethanol. |
|
|
|
|
An
old-fashioned name for ethene |
|
|
|
|
Systematically
called ethanediol, a colourless viscous hygroscopic liquid used in antifreeze
and in wood preservatives. |
|
C2H6O2 |
|
|
The first
member of the alkyne homologous
series, formerly known as acetylene. |
|
C2H2 |
|
|
The
old-fashioned name for methanoic acid. |
|
|
|
|
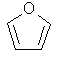
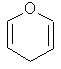
One half
of the 80s chemical pop sensation Furan Furan. |
|
C4H4O |
|
|
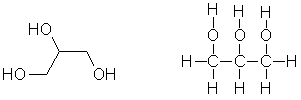
Systematic
name called propane-1,2,3-triol. The molecule at the heart of fats and oils,
this trihydric alcohol is also used to make bubble mixture and in the catering
industry. It is sometimes referred to
as glycerine. |
|
C3H5(OH)3 |
|
|
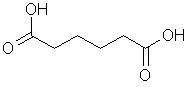
Mixed with
hexanedioic acid to make nylon(6,6). Also
called 1,6-diaminohexane. |
|
C6H16N2 |
|
|
Mixed with
hexane 1,6-diamine to make
nylon(6,6). |
|
C6H14O4 |
|
|
Compounds
containing only hydrogen and carbon atoms. |
|
CXHY |
|
|
The
simplest carboxylic acid possible. |
|
HCOOH Also: CH2O2 |
|
|
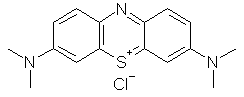
An
indicator with many other uses including staining cells in biology and
related sciences, and as a medicinal drug. |
|
C16H18ClN3S |
|
|
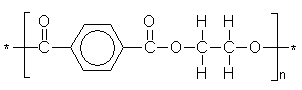
PolyEthylene Terephthalate, a polymer used to make drinks bottles, x-ray
films, audio tapes and many other products.
As an artificial fibre it is known as Terylene® and Dacron®. |
|
(C10H8O4)n |
|
|
|
|
C5H10O |
|
|
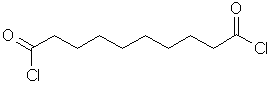
A diacyl
dichloride also known as decanedioyl
dichloride. It is used in Salters
Advanced Chemistry Activity DP2.1 Making Nylon (click
to view details of this activity). |
|
C10H16Cl2O2 |
|
|
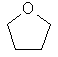
A
clear colourless liquid which smells like ether and dissolves easily in
organic solvents and water. |
|
C4H8O |
|
|
Click on an orange label to see a hazcard |
||||
|
A brick
red powdery solid, formed when copper(II) ions (Cu2+) are reduced
in alkaline aqueous solutions to copper(I) ions (Cu+). |
Crystal lattice structure |
Cu2O |
||
|
The more
common oxide of copper. |
Crystal lattice structure |
CuO |
||
|
An
explosive consisting of a powdered mixture of saltpetre, sulphur and
charcoal. |
Mixture |
KNO3, S, C |
||
|
A soft
white or grey mineral consisting of hydrated calcium sulphate, used to make
plaster of Paris and in the building industry. |
|
CaSO4.2H2O |
||
|
A powerful
acid used to etch glass, this liquid is extremely dangerous. As an
anhydrous compound, hydrogen fluoride is a viscous, fuming liquid. Unlike hydrogen chloride it is not a gas at
room temperature due to the very strong hydrogen bonding between neighbouring
molecules. This
arises because of fluorine’s uniquely high electronegativity. The structure to the right shows that the
fluorine atom strongly attracts electrons in the hydrogen-fluorine covalent
bond, resulting in a massive dipole effect. Although
technically a weak acid, HF is highly dangerous as it destroys biological
tissues at a frightening rate, penetrating deep into human flesh. If you spill HF on your hand, it may have
etched away down to the bone before you realise anything is wrong. Calcium gluconate gel can be used in
treatment. |
+δH—Fδ- |
HF |
||
|
Another
word for the hydroxonium
ion. |
|
|
||
|
The ion
formed when protons dissociate from their parent acid molecule in aqueous
acid solutions. Click here for more
details. |
|
H3O+ |
||
|
Another
word for the hydroxonium
ion. |
|
|
||
|
The anion
found in phosphoric acid. It also
makes up part of the backbone of DNA and RNA, interspersed by ribose sugar
units. |
|
PO43- |
||
|
A highly
poisonous solution containing cyanide (CN-) ions. |
|
KCN |
Extremely
toxic |
|
|
Powerful
reducing agent used in organic synthesis. NaBH4 is a
source of hydride ions (H-) which act as reducing agents by adding
hydrogen to organic compounds. As a quick
recap of OIL RIG, oxidation is: ·
loss
of electrons ·
gain
of oxygen ·
loss
of hydrogen Reduction
is: ·
gain
of electrons ·
loss
of oxygen ·
gain
of hydrogen It is less
reactive than the more powerful reducing agent lithium
tetrahydridoaluminate(III) (also known as lithium aluminium hydride) which
means it is safer to use. The +3
oxidation state of boron indicated in the name is a bit of a pointless piece
of information to include because boron has a +3 oxidation state in all its
compounds. Synonym:
sodium borohydride. |
|
NaBH4 |
||
|
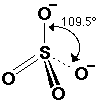
[sulphate(VI)] |
Although
this diagram shows double bonds and two single bonds, in real life each
sulphur-oxygen bond is identical. Four
of the electrons in the double bonds in the picture are actually spread out
equally between all the atoms. They
are delocalised over the whole ion, just like the double bonds in benzene. |
|
SO42- |
|
|
The anion
O2- |
|
O2- |
||
|
A salt in
which the anion contains both tungsten and oxygen. Tungstate (VI) has the formula WO42-. |
|
WO42- |
||
|
The
chemical element of atomic number 74, a hard steel-grey metal with a very
high melting point (3410°C), used to make electric light filaments. |
Metallic lattice structure |
W |
||
|
A very
hard grey compound made by reaction of tungsten and carbon at high
temperatures, used in making engineering dies, cutting and drilling tools,
etc. |
Crystal lattice structure |
WC |
||
|
A yellow
mineral consisting of hydrated tungsten oxide, typically occurring as a
powdery coating on tungsten ores. |
Crystal lattice structure |
WO3.H2O |
||
|
A highly
polar molecule, liquid at room temperature, formed when hydrogen gas (H2)
burns in air or in pure oxygen. Liquid
water exists almost entirely as covalent water molecules. Its pH is 7, which corresponds to a
concentration of H+(aq) ions of only 10-7
mol dm-3. In
comparison, a strong acid such as hydrochloric acid has a pH of about 1,
which corresponds to a [H+(aq)] of 10 mol
dm-3, which is one hundred million times more than water. |
|
H2O |
||
|
A
synthetic drug developed in the 1890s used to relieve pain and reduce
fever. It is derived from salicylic
acid, which itself comes from salicin, a compound found in willow trees. Origin of name: aspirin was discovered by Felix
Hoffman, a German chemist. The German
name for the compound is acetylierte
Spirsäure (acetylated salicylic acid).
This became shortened to aspirin. |
|
C9H8O4 |
|
|
Mr = 180 |
|||
|
Synonyms: 2-ethanoylhydroxybenzoic acid acetylsalicylic acid |
|||
|
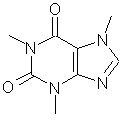
A drug
found in tea, coffee, chocolate and Coke.
Caffeine
gives people a lift and helps them to be mentally alert. The drug is used medically to stimulate the
nervous, respiratory and cardiovascular systems. Caffeine
is also added to medicines to counteract sleepiness caused by other
ingredients. |
|
C8H12N4O2 |
|
|
Mr = 182 |
|||
|
Synonyms: 1,3,5-trimethylxylene |
|||
|
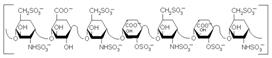
A compound
occurring in the liver and other tissues which inhibits blood coagulation,
used as an anticoagulant in the treatment of thrombosis. Origin of name: from Greek hepār (liver) + in (an
ending used for organic compounds, pharmaceutical products, proteins and so
on) |
|
|
|
|
Mr
= 6000 to 40000 |
|||
|
|
|||
|
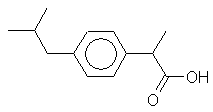
An
anti-inflammatory and analgesic drug sold over the counter. Origin of name: certain parts of the name
2-(4-isobutylphenyl) propionic acid. |
|
C13H18O2 |
|
|
Mr = 206 |
|||
|
Synonyms: 2-(4-(2-methylpropyl)phenyl
propanoic acid 2-(4-isobutylphenyl)
propionic acid |
|||
|
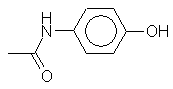
A
synthetic drug used to relieve pain and reduce fever and swelling. Paracetamol is regularly used in hospitals
and is sold over the counter by pharmacists. Origin of name: one name for the structure of the
compound is paraacetylaminophenol. In the 1950s this became shortened to
paracetamol. |
|
C8H9NO2 |
|
|
Mr = 151 |
|||
|
Synonyms: acetaminophen (USA) |
|||
|
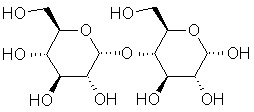
A sugar
produced by the breakdown of starch, sometimes by enzymes found in malt. |
|
C12H22O11 |
|
|
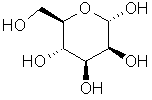
A sugar
which occurs as a component of many natural polysaccharides and is part of
the hexose class of sugars. |
|
C6H12O6 |
|
|
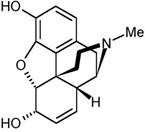
morphine |
Powerful
painkiller related to heroin. Me
stands for –CH3. |
|
|
Hydrolysis
that is carried out in an acidic solution. |
|
|
Zero
Kelvin (0 K) or -273.15 °C which is the lowest temperature theoretically
possible. At this temperature atoms do
not vibrate at all, resulting in no heat.
It is impossible to achieve absolute zero in real life, but it is
possible to get very close (a few millionths of a degree above). |
|
|
A measure
of the capacity of a substance to absorb light of a specified wavelength,
often used in colorimetry. It is
given in terms of I0 (intensity of light at source) and I
(intensity of light after passing through substance) by this equation: A = -log10(I/I0) It is also given by Beer’s Law A = εℓc where ε is molar absorptivity, ℓ is path length of light and c is concentration. If Greek
letters such as ε confuse or annoy you, try looking at my sheet about
the Greek alphabet. |
|
|
(When
relating to an organic compound) containing a planar unsaturated ring of
atoms which is stabilised by an interaction of the bonds forming the ring. |
|
|
The
reverse process of synthesis, where a large molecule is broken down into
smaller, simpler molecules. |
|
|
A process
where a large molecule is broken down into simpler ones. |
|
|
To become
liquid by absorbing moisture from the air (talking about a solid). |
|
|
A lepton
with a charge of -1 relative to the charge on a proton. The charge on an electron is 1.6 x 10-19
Coulombs. |
|
|
a red
blood cell |
|
|
an enzyme
which breaks down particular esters into acids and alcohols or phenols |
|
|
Water or water-related
(like in hydrolysis) or it can mean hydrogen (like in hydrochloric acid or
hydrocarbon). Hydro- came directly
from the Greek word for water hudron. |
|
|
A
reaction where a molecule is split by water. |
|
|
A
substance that tends to absorb moisture from the air is hydroscopic. |
|
|
An eye
irritant. |
|
|
Decomposition. I like to think of it as meaning
splitting. It came from the ancient
Greek word lusis
which means loosening. It’s easy to
see how loosening is similar to decomposition – when something loosens it is
held less strongly. When the knots in
a net loosen the net decomposes, it falls apart. Electrolysis
means chemical decomposition by electric current. The electrons holding a compound together
are loosened and let free by adding more electrons from an electric
current. As a result the compound
decomposes. Catalysis
means using a catalyst to accelerate a chemical reaction. The word came from kataluein, a Greek verb meaning
dissolve, which in turn is made up of kata
(down) and luein
(loosen). This is an example of a
word where it’s harder to see the connection between its constituent parts
and its meaning. Hydro-
means water and glyco- means glucose. Hydrolysis
means chemical breakdown due to reaction with water and glycolysis means the
breakdown of glucose by enzymes. The
part of the word before –lysis can refer to either the thing causing the
splitting (water) or the thing being split (glucose). |
|
|
Containing
the alkyl radical –CH3 which comes from methane. |
|
|
para- |
Complicated: denoting substitution at
diametrically opposite carbon atoms in a benzene ring, for example paradichlorobenzene Simple: when you have two groups attached
to a benzene ring, there are three different isomers possible. The para- isomer is the one where the two
groups are opposite each other, and are as far away from each other as
possible. The other isomers are called
ortho- (next-door neighbours) and meta- (separated by one notch). Important note: para-, meta- and ortho- are not
standard IUPAC-approved ways of describing the structure of chemicals. They are getting a bit outdated and most
newly-published books will use the systematic IUPAC nomenclature (naming
system). Even though IUPAC is trying
to get everyone to speak their new language, many older documents and some
new ones still use the old fashioned names.
For one thing, the names look ridiculous using the systematic nomenclature:
paradichlorobenzene becomes 1,4-dichlorobenzene. Who wants to be faffing around with all
those numbers? Apparently they’re
supposed to remove any ambiguity, but that’s debatable.
Origin of the word: the prefix comes from the Greek
word para (παρα),
meaning beside or beyond. Not to be
confused with the Northampton slang para,
a shortened form of paralytic,
meaning extremely drunk. Paralytic comes from paralysis – because it is possible
drink so much that you can’t stand up, at which point you have achieved at
least one symptom of paralysis.
Although many people will testify that this is true, getting para is
not recommended. (Included for legal reasons only). |
|
(Adjective)
of or relating to medicinal drugs, or their preparation, use or sale (Noun) a
compound manufactured for use as a medicinal drug Origin of the word: via late Latin from Greek pharmakeutikos
[from pharmakeutēs (druggist) from pharmakon (drug)]. |
|
|
The branch
of medicine concerned with the uses, effects and action of drugs. |
|
Origin of the word: via medieval Latin and Old French farmacie from Greek pharmakeia (φαρμακεια)
meaning practice of the druggist, based on pharmakon (φαρμακον)
meaning drug. |
|
|
To cool a
liquid below its freezing point without solidification or crystallization. |
|
|
The
process of making a new substance from simpler substances. Compare with degradation. |
|
|
The
branch of chemistry concerned with the quantities of heat evolved or absorbed
during chemical reactions. |
|
|
Readily
destroyed or deactivated by heat.
Compare with thermostable. |
|
|
The
breakdown of molecules by the action of heat. |
|
|
Not
readily destroyed or deactivated by heat.
Compare with thermolabile. |
|
|
Sticky or
thick, like treacle. |