The crystal structure of fluorite, CaF2
Wikipedia: Calcium fluoride, Fluorite
Housecroft: p. 168
Shriver & Atkins: pp. 86–87
Smart & Moore: pp. 35 37
Greenwood & Earnshaw: pp. 117–118
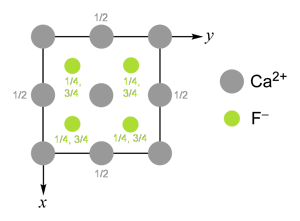
The fluorite structure is the ideal structure of calcium fluoride, CaF2.
The structure can be envisaged as a cubic "close packed" array of cations with all tetrahedral holes filled by anions.
Ca2+ is cubically coordinated by eight F−, whereas F− is tetrahedrally coordinated by four Ca2+.
|
|
Page skeleton and JavaScript generated by export to web function using Jmol 11.6.8 2008-11-24 13:39 on Mar 25, 2009.
