The nickel arsenide (NiAs) crystal structure type
Wikipedia: Nickeline
Housecroft: p. 169
Shriver & Atkins: pp. 86–87
Smart & Moore: pp. 33 35
Greenwood & Earnshaw: pp. 679, 1209
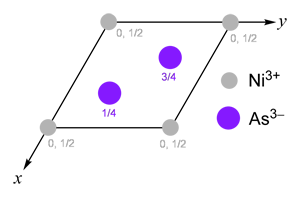
The nickel arsenide structure can be thought of as a hexagonal close packed array of anions, with all the octahedral interstices filled by cations.
Every anion is surrounded by four cations and has tetrahedral coordination geometry. Likewise, every cation is tetrahedrally coordinated by four anions.
The nickel arsenide structure type is exceptionally common in compounds with MX stoichiometry, with only the NaCl structure having more examples, but is generally limited to compounds of transition metals with As, Sb, Bi, S, Se, Te and occasionally Sn. Compounds adopting the NiAs structure include NiS, NiSb, FeS and CoS. PtB has the anti-NiAs structure.
|
|
Page skeleton and JavaScript generated by export to web function using Jmol 11.6.8 2008-11-24 13:39 on Mar 25, 2009.
