The crystal structure of caesium chloride, CsCl
Wikipedia: Caesium chloride
Housecroft: pp. XX
Smart & Moore: pp. XX
Greenwood & Earnshaw: pp. XX
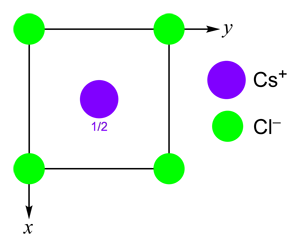
The caesium chloride structure is also known as the CsCl structure. One way to understand it is to consider it as a primitive cubic array of anions, with all the cubic interstices filled by cations.
Every anion is surrounded by eight cations and has cubic coordination geometry. Likewise, every cation is cubically coordinated by eight anions.
Compounds with large cations tend to adopt the CsCl structure, most notably CsBr, CsI, CsCN, [NH4]Cl, [NH4]Br, TlCl, TlBr, TlI and the intermetallics CuZn, CuPd and LiHg.
For any symmetry fans out there, CsCl adopts the Pm-3m space group (No. 221).
|
|
Page skeleton and JavaScript generated by export to web function using Jmol 11.6.8 2008-11-24 13:39 on Mar 25, 2009.
