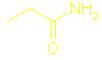
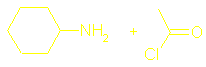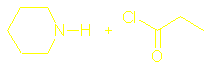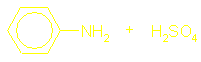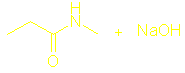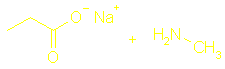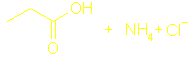Section 13.8 Amines and amides
Slightly
relevant fact: amines and amides get their name from am- in ammonia
Highly
irrelevant fact: ammonia gets its name from sal
ammoniac, an outdated word for ammonium chloride NH4Cl. The name came from sal ammoniacus, Latin for ‘salt of Ammon’. The word ammoniacus
came into the Latin vocabulary from Greek, in the form of the Greek word ammōniakos (αμμωνιακος),
meaning ‘of Ammon’.
Shockingly
pointless fact: What’s Ammon and why is ammonium chloride named after it, you ask? The salt found by the Greeks near the
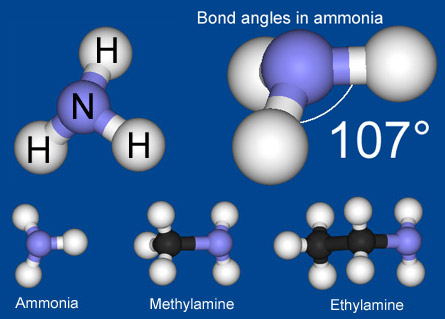
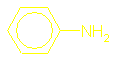
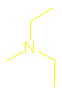
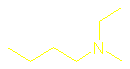
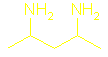
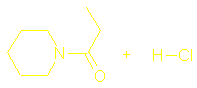
What are amines and how are they named?
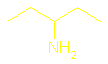
CH3–CH(NH)
–CH2–CH2–CH3
is called 2-aminopentane.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Properties of amines
The lone pair of electrons explains why
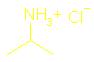
amines and ammonia are: ·
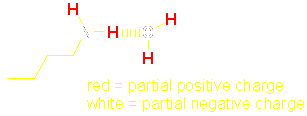
very soluble in water ·
a base ·
a Ligand ·
a nucleophile Solubility of amines
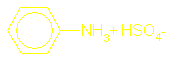
Amines as bases
Amines as ligands
There should be a paragraph on amines as nucleophiles but I haven’t
written it yet. It’s on page 332 of
Chemical Ideas.
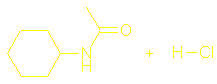
There should
also be sections on What are amides? Hydrolysis of amides and Condensation polymers involving the NH2 group but I couldn’t
be bothered to write them when I made this page. Hopefully
I’ll update it sometime. Watch this
space. It’s all on p332-334 of
Chemical Storylines. At least I’ve
given you the answers to the questions so you can pretend you did them for
homework. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Problems for 13.8
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||