Polymers,
Proteins and Steel
sketchy Exam Revision
Recrystallisation
Questions
about recrystallisation are worth about
3-4 marks each, but often have an extra mark for the quality of your written
communication, so they’re well worth getting right.
1. Dissolve your impure
crystals in the minimum possible amount of solvent. The solvent is usually water. Gently heat the solution until all the crystals
have dissolved.
1. Leave the solution to
cool very slowly, perhaps overnight.
2. If any debris remains,
decant most of the clear solution from the debris into a second flask. Then heat the solution again to redissolve
the crystals.
3. Cover and leave to recrystallise.
4. Collect the crystals
by vacuum filtration and leave them to dry on a watch-glass. You can speed the process up by placing it in
an oven or on a hotplate.
The
gist of it is…
v dissolve impure solid in minimum of solvent
v cover and leave to cool
slowly
v vacuum filter the
crystals and leave to dry
Hydrolysis
Hydrolysis
is the splitting of amide and peptide linkages into their original
constituents. It’s the reverse process
of condensation reactions.
·
Dipeptides can be hydrolysed
into their two constituent amino acids
·
Condensation
polymers can be hydrolysed into the constituents monomers, especially nylon(6,6) →
1,6-diaminohexane + hexanedioic acid (DP2.1)
·
Hydrolysis takes
place best under reflux, warmed, for several hours, with moderately
concentrated (4M-6M) hydrochloric
or sulphuric acid or sodium hydroxide pretty much like in
your stomach
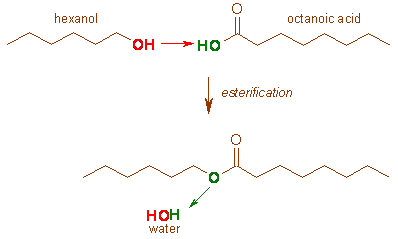
Esterification
Esters
(RCOOR’) are formed when alcohols react with carboxylic acids.
Rusting
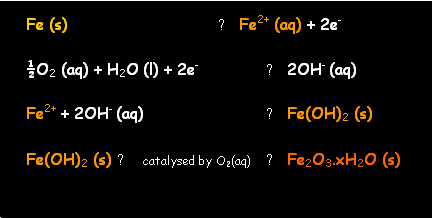
·
Iron(0) is oxidised to iron(II) ion
·
Oxygen and water are
reduced to hydroxide
·
Iron(II) hydroxide
forms as a precipitate
·
Iron(II) hydroxide
is oxidised to iron(III) oxide
Overall
iron undergoes two-stage oxidation from Fe to Fe2+ to Fe3+. Both water and dissolved oxygen are
required. The dissolved oxygen
concentration is greatest near the outside of the droplet, so electrons flow here
from the iron being oxidised to iron(II) ions in the centre of the droplet
where the [O2] is lowest. At
the outside,
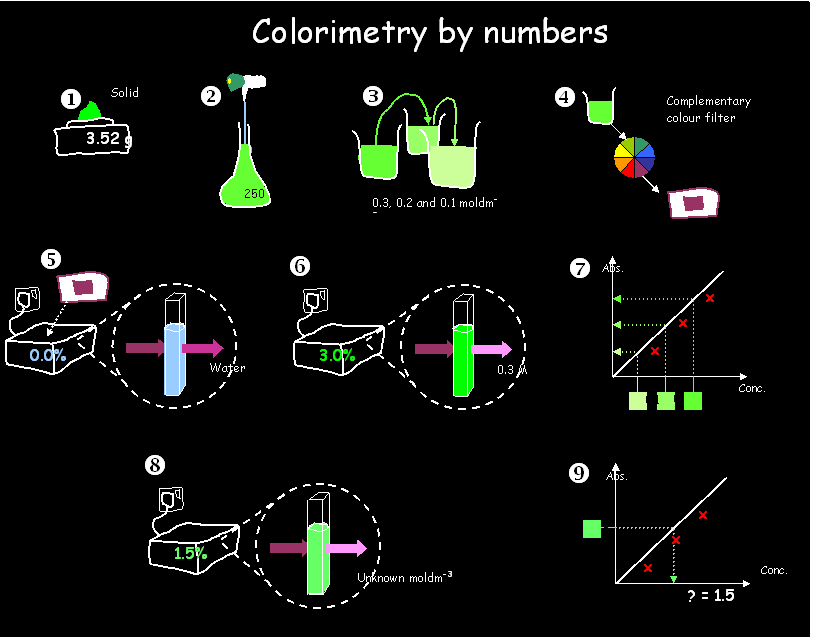
Colorimetry
There
is often a question about colorimetry in the PP&S exam, and it usually
carries 4 or 5 marks, around 5% of the total.
You are given a sample solution of [some chemical] of unknown
concentration, and some pure, solid [chemical]. You have to find the
concentration of the unknown sample by colorimetry. The
more intensely the unknown sample absorbs
a colour of light, the more
concentrated it is.
·
Weigh the solid to
find its mass
·
Dissolve the solid
in distilled water up to a precise
volume
·
Make up a range of solutions of different known concentrations by serial dilution
·
Choose an appropriate filter of a complementary colour to the solution
·
Use a blank reference cell filled with water
to zero the colorimeter
·
Measure and record the absorption of the solution at your range of concentrations
·
Plot a calibration curve of concentration vs. absorption
·
Measure and record the absorption of the solution
of unknown concentration
·
Read off the
sample’s concentration, from its
absorption, on the calibration curve
Complexes
·
Ligands are molecules or ions which surround a positively-charged central metal ion and form
(dative/coordinate) covalent bonds with
it.
·
A ligand, by
definition, has at least one lone pair of electrons, which it needs
to donate to the metal ion form the dative covalent bond.
·
Ligands are given generic names based on how many points of attachment they
contain. A general rule of thumb is that
any atom in the ligand molecule with a lone pair of electrons can be a point of
attachment, although this is not true every time. In the following table, a colon ( : ) means a lone pair of electrons.
|
Points of
attachment |
Name of class of ligand |
Example |
|
1 |
Monodentate |
water, H2O:, ammonia :NH3 |
|
2 |
Bidentate |
ethanedioate, 1,2-diaminoethane |
|
6 |
Hexadentate |
edta4-, 4O: and 2N: |
|
more than one |
Multidentate |
many, mostly organic, O: & N: |
·
The combined molecule made of a central
metal ion surrounded by ligands is called
a complex. You might have to state the co-ordination number and name one in the exam so here’s how:
1.
First, how many ligands are attached? The number attached is called the co-ordination number.
2.
Secondly, in alphabetical order, identify the molecules
acting as ligands by their special
ligand names. Anions (negative ions)
swap –ide for –o at the end of their names and neutral ligands keep the same
name, except water and ammonia because they’re very common.
|
How many ligands |
Prefix |
|
Ligand |
Special name |
|
1 |
Mono |
|
Cl- |
chloro |
|
2 |
Bi |
|
CN- |
cyano |
|
3 |
Tri |
|
F- |
fluoro |
|
4 |
Tetra |
|
|
hydroxo |
|
5 |
Penta |
|
H2O |
aqua |
|
6 |
Hexa |
|
NH3 |
ammine |
3.
Thirdly, name the central metal ion. Use the English name if the complex has an overall positive
or neutral charge and use the Latin
name if the complex is overall negatively
charged.
4.
Show the oxidation
number of the central metal ion in brackets in roman numerals.
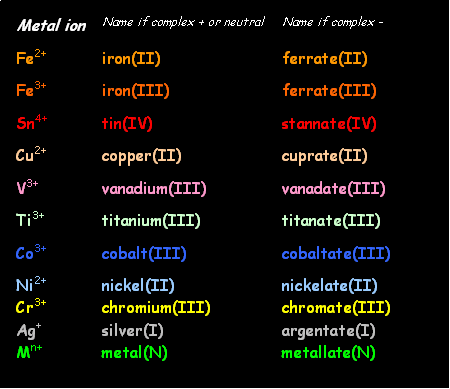
5.
Combine stages 1-4
to make a whole name, such as hexaaquacopper(II) =
[Cu(H2O)6]2+.
Further
examples follow:
·
[Fe(CN)6]4-
= hexacyanoferrate(II)
·
[CuCl4]2-
= tetrachlorocopper(II)
·
[Ni edta]2- = mono-edta-nickelate(II)
·
[FeCl3(NH3)3]
= triamminetrichloroiron(II)
Basic Oxygen Steelmaking process
Industrial
production of steel is a batch process.
Impurities in the blast furnace are removed with oxygen gas and basic
oxides, hence BOS.
·
Mg (s) + S (s) → MgS (s)
·
Excess carbon,
silicon, manganese and phosphorus impurities are removed by reaction with
oxygen gas, unavoidably along with some iron
Si (s) + O2 (g) → SiO2 (s)
Mn (s) + O2 (g) → MnO
(s)
4P (s) + 5O2 (g) → P4O10
(s)
C (s) + O2 (g) → CO2 (g)
2Fe (s) + O2 (g) → 2FeO (s)
·
Acidic oxides SiO2
and P4O10 are removed by basic oxides CaO and MgO.
SiO2 +
CaO → CaSiO3 calcium
silicate (component of slag)
SiO2 +
MgO → MgSiO3
P4O10
+ 2MgO → 2Mg3(PO4)2
P4O10
+ 2CaO → 2Ca3(PO4)2