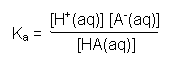Constants
& Equations
|
Equation |
Name & Meaning |
Notes |
|
E = hf |
Planck’s equation Photon energy = Planck’s
constant × photon frequency Question If an electron changes
energy levels from 5.00 × 10-20 Joules to 4.00 × 10-20
Joules, and transfers the energy it loses to a photon, what will be the
frequency of the photon? Answer The change in energy of
the electron, ΔE =
(4.00 – 5.00) × 10-20. ΔE =
-1.00 × 10-20 J. The change in energy is negative because the electron loses energy. The photon emitted will gain the energy
lost by the electron. Therefore the energy of
the photon is the same value and the opposite sign of the change in energy of
the electron: E = -ΔE (of electron). E = - (-1.00 × 10-20) = 1.00 × 10-20 J Use Planck’s equation: E = hf Divide both sides by h to make frequency the subject of
the expression: E / h = f Enter the values of E and h into the expression, then use a calculator to find the answer: f = E
/ h = (1.00 × 10-20) / (6.63 × 10-34) = 1.51 × 1013 Hz |
Planck’s constant, h = 6.63 × 10-34 JHz-1 Frequency may be
represented by the letter f or by
the Greek letter ν (called
Nu, pronounced noo) – if ν is
used, the expression becomes E = hν. It still has the same meaning. |
|
Ka |
Acid dissociation constant
HA is a weak acid (only a
small percentage of its molecules dissociate into hydrogen ions and anions). It dissociates into a hydrogen ion H+
and its conjugate base A-. |
The acid dissociation
constant for weak acids is a special case of an equilibrium
constant. It is derived from the
expression for Kc and the equilibrium reaction HA(aq)
|
|
Kw |
The ionic product of water Kw = [H+(aq)]
[ Kw = 1.00 × 10-14
mol2dm-6 at 298K Ionic product of water =
concentration of hydrogen ions × concentration of hydroxide ions (All concentrations in
moles per litre, mol dm-3) |
The ionic product of water
is a special modification of an equilibrium constant, called the acid
dissociation constant of water. The acid dissociation
constant of water is a more complicated expression than is necessary because
the concentration of water can be treated as constant as it is always in
excess. |