Infrared spectroscopy
A quick reminder about infrared radiation
Infrared radiation is electromagnetic radiation which occupies the region of the electromagnetic spectrum between microwaves and visible light.
Infrared spectroscopy is the study of the interaction of infrared raditation with matter as a function of wavelength. An infrared spectrum shows how strongly a sample of matter absorbs each wavelength of IR radiation.
There's a really good set of notes online about Spectroscopy in A-level Chemistry by Dr Nick Greeves from the University of Liverpool.
IR spectroscopy has many applications, including identifying functional groups in organic compounds. It is this application that receives the most attention in A-level Chemistry courses.
The infrared spectrum
I have found a variety of different definitions of the infrared region of the electromagnetic spectrum, including one from the IUPAC Gold Book. The boundaries given below are from Atkins' Physical Chemistry, 7e. The infrared part of the EM spectrum can be subdivided into near, mid and far regions. Near infrared is so called because it is nearest to the visible part of the EM spectrum, while far IR is furthest away from visible.
The IR spectrometers I've used in my undergraduate laboratory work usually record the IR absorption spectrum of a sample from about 400 cm−1 to 4000 cm−1, which approximately corresponds to the mid infrared region.
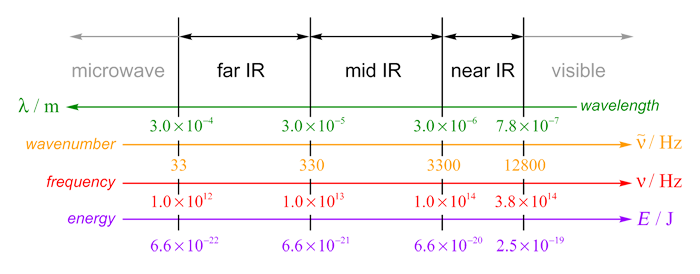
| Region | Wavelength / m | Wavenumber / cm−1 | Frequency / Hz | Energy / J |
| Near infrared | 7.8 × 10−7 – 3.0 × 10−6 |
13 000 – 3300 |
3.8 × 1014 – 1.0 × 1014 |
2.5 × 10−19 – 6.6 × 10−20 |
| Mid infrared | 3.0 × 10−6 – 3.0 × 10−5
|
3300 – 330 |
1.0 × 1014 – 1.0 × 1013 |
6.6 × 10−20 – 6.6 × 10−21 |
| Far infrared | 3.0 × 10−5 – 3.0 × 10−4 |
330 – 33 |
1.0 × 1013 – 1.0 × 1012
|
6.6 × 10−21 – 6.6 × 10−22 |
Why do molecules absorb infrared radiation?
Atoms and molecules have several different kinds of quantised energy levels, each of which can interact with different forms of electromagnetic radiation:
-
Atoms and molecules translate and can undergo translational transitions from one translational energy level to another
-
Molecules rotate and can undergo rotational transitions from one rotational energy level to another
-
Molecules vibrate and can undergo vibrational transitions from one vibrational energy level to another
-
Atoms and molecules have electrons which can undergo electronic transitions from one electronic energy level to another
Atoms and molecules can translate (move as a whole in a specific direction) and both have electrons which occupy electronic energy levels. Only molecules can rotate or vibrate, because unlike atoms, molecules are not spherically symmetric.

You need a spectrometer to infrared spectroscopy
This year (2008-9), there are new laser Fourier transform IR spectrometers in the teaching labs at the University of Bristol's School of Chemistry. In the image to the right, they grey and pale blue plastic box is the spectrometer, which is connected to a PC to view, record, save and print spectra. You control the spectrometer using software on the PC. To record the IR spectrum of a solid sample, you put some powdered solid on the steel disc, click the movable arm into position above the disc, then tighten the screw top until it is squeezing your powder hard enough (there's a pressure meter on the PC). You then click a button and it takes about 30 seconds to record four spectra and average them.
Infrared spectroscopy shows which functional groups are present in an organic molecule
IR spectroscopy is routinely used by chemists to find out what they have made. The technique doesn't tell you everything about the structure of your molecule, but it's really good at identifying the presence or absence of many functional groups, especially O-H and C=O groups.